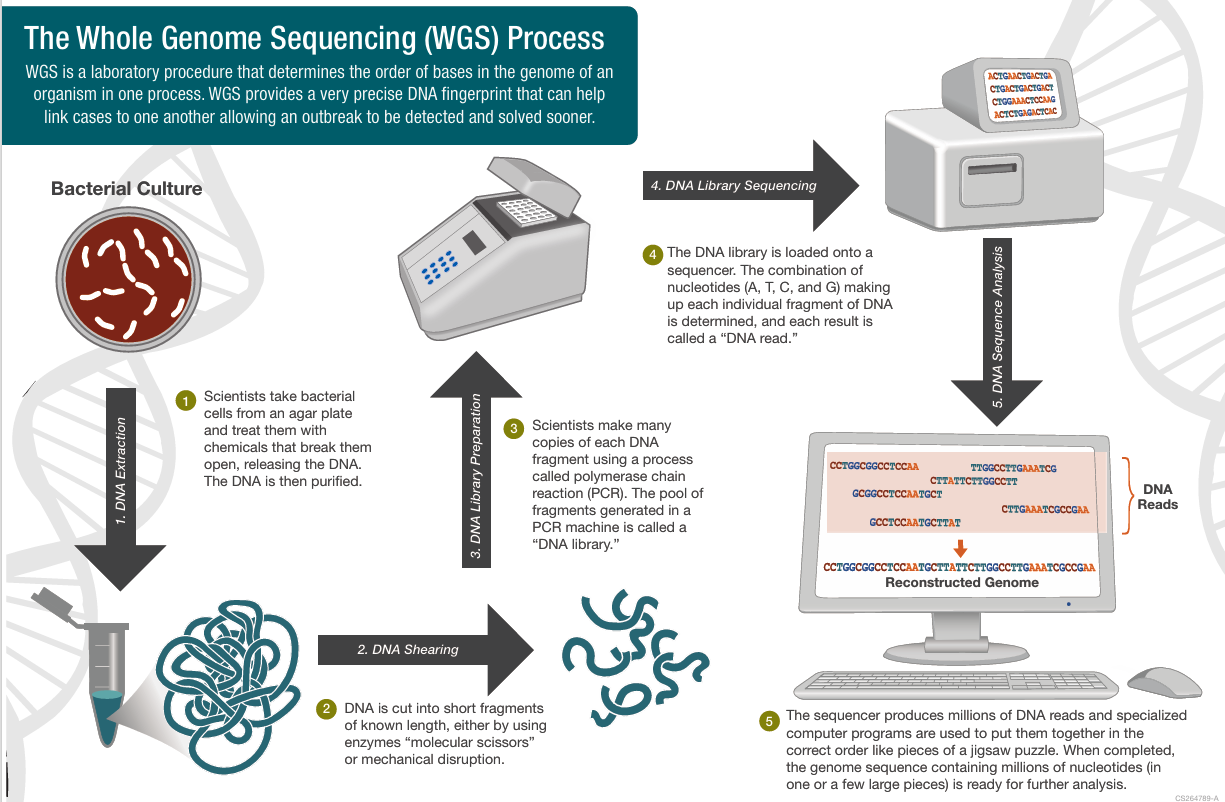
Introduction
Britain is on the brink of a scientific milestone: the largest-ever genetic sequencing release from its own UK Biobank. This massive dataset will include genetic sequences from millions of volunteers, dwarfing all past releases. Such an unprecedented endeavor promises to accelerate genomic research, help unlock the causes of complex diseases, and pave the way for personalized medicine. But it also raises questions about data privacy, ethical use, and how researchers worldwide will harness this trove of genetic information. In this article, we explore the background of the UK Biobank, the scope of the upcoming release, its potential impact, and the challenges that lie ahead.
The UK Biobank: A Treasure Trove of Genetic Data
Origins and Mission
Launched in 2006, the UK Biobank set out to improve prevention, diagnosis, and treatment of diseases by gathering extensive health and genetic data from 500,000 adult volunteers across the UK. Participants contributed medical records, lifestyle questionnaires, physical measures (like height and blood pressure), and blood samples. This broad approach created a richly detailed resource linking genetics with health outcomes.
Early Sequencing Efforts
Over its first decade, the UK Biobank made waves by releasing genotyping arrays—mapping hundreds of thousands of genetic markers. In 2017, it began whole-exome sequencing for 50,000 participants, revealing mutations in protein-coding regions. Each data release fuelled hundreds of research studies into conditions like diabetes, heart disease, and dementia.
The Largest-Ever Release: What’s New?
Scale and Scope
The upcoming release, scheduled for early 2026, will include whole-genome sequences for up to three million participants—six times larger than any previous public dataset. Unlike exomes, whole-genome sequencing reads all three billion base pairs of DNA, capturing non-coding regions that regulate gene activity.
Data Types
- Raw Sequences: Complete A-C-G-T data for each genome.
- Variant Calls: Lists of mutations and structural changes compared to a reference genome.
- Phenotype Links: Anonymized health records and lifestyle data associated with each sequence.
- Quality Metrics: Confidence scores indicating the accuracy of each read.
Open-Access Model
To maximize global impact, the UK Biobank will maintain its open-access policy. Qualified researchers can apply to use the data for health-related studies, with strict privacy safeguards and governance protocols. This model promotes collaboration across academia, industry, and public health.
Potential Impact on Medicine and Science
Accelerating Disease Gene Discovery
By comparing genomes of healthy versus affected individuals, researchers can pinpoint genetic variants that raise disease risk. A dataset of this size will:
- Reveal rare mutations that occur in fewer than 1 in 10,000 people.
- Clarify how non-coding regulatory regions influence gene expression.
- Uncover gene–environment interactions, such as how diet or pollution modify genetic risk.
These insights could lead to new drug targets and early diagnostic tests.
Personalized Medicine
Whole-genome data combined with medical records allow doctors to tailor treatments to each patient’s genetic profile. Examples include:
- Pharmacogenomics: Predicting which drugs a patient will metabolize safely and effectively.
- Risk Stratification: Identifying individuals at high genetic risk for conditions like breast cancer or Alzheimer’s disease, enabling preventive strategies.
- Gene Therapy Development: Designing therapies that correct harmful mutations at the DNA level.
Public Health and Population Genetics
The UK Biobank’s rich demographic data enable researchers to study genetic variation across age groups, ethnic backgrounds, and geographic areas. This can inform:
- Population Screening: Cost-effective programs to test for hereditary conditions.
- Vaccine Response: Genetic factors that affect how well people respond to vaccines or infections.
- Health Inequalities: Understanding genetic and environmental contributions to disparities in disease prevalence.
Ethical, Privacy, and Legal Considerations
Participant Consent and Data Protection
All UK Biobank volunteers provided broad consent, agreeing their data could be used for “health-related research” indefinitely. Yet whole-genome sequences carry more sensitive information than genotyping arrays. The Biobank employs:
- De-identification: Removing names, addresses, and direct identifiers.
- Controlled Access: Researchers must apply, justify their project, and sign data-use agreements.
- Secure Infrastructure: Data stored on protected servers with audit trails to track usage.
Balancing Openness and Security
While open access speeds discovery, it also increases risk of re-identification. The Biobank relies on a tiered access model:
- Aggregate Data: Publicly available summary statistics.
- Sensitive Data: Requires special approval and monitoring.
- Third-Party Oversight: Ethical review committees vet applications and enforce penalties for misuse.
Equity and Representation
Most participants in early releases were of European ancestry, limiting generalizability. The UK Biobank is striving to include more diverse volunteers through targeted recruitment in underrepresented communities, ensuring findings benefit all.
Preparing Researchers for the Data Deluge
Training and Education
Discovering insights from millions of genomes demands new skills. Universities and institutes offer:
- Workshops on Bioinformatics Tools: Learning pipelines for sequence analysis, variant calling (e.g. GATK), and genome assembly.
- Cloud Computing Courses: Using AWS, Google Cloud, or Microsoft Azure to process large datasets.
- Ethics and Data Governance Seminars: Understanding patient privacy, consent, and legal frameworks.
Building Scalable Infrastructure
Research centers invest in:
- High-Performance Clusters (HPCs): Thousands of CPU cores for parallel processing.
- Data Lakes: Centralized storage that integrates genomic, clinical, and environmental data.
- Open-Source Pipelines: Community-developed workflows (Nextflow, Cromwell) for reproducible research.
By sharing best practices, the global community can avoid duplicated effort and accelerate breakthroughs.
Collaboration and Global Partnerships
International Consortia
The UK Biobank’s data will feed into global efforts like:
- The Global Alliance for Genomics and Health (GA4GH): Establishing data standards and sharing protocols.
- All of Us Research Program (U.S.): Combining data to study cross-population genetic effects.
- European Genome-Phenome Archive (EGA): Enabling multi-country meta-analyses.
Public–Private Partnerships
Pharmaceutical companies and biotech startups partner with academic researchers to:
- Develop new diagnostics based on genetic markers.
- Screen drug libraries using AI models trained on Biobank data.
- Launch clinical trials targeted to genetically defined subgroups.
These collaborations can speed drug discovery while adhering to ethical guidelines.
Looking Ahead: Challenges and Opportunities
Managing Big Data Complexity
Even with robust infrastructure, analyzing millions of whole genomes presents challenges:
- Variant Interpretation: Distinguishing harmful mutations from benign variants.
- Computational Costs: Cloud and HPC resources can be expensive—sustainable funding models are needed.
- Data Integration: Linking genetic data with lifestyle, environmental exposures, and electronic health records.
Innovations in AI and machine learning—such as deep learning for variant effect prediction—offer solutions but require careful validation.
Ensuring Equitable Benefits
To avoid widening health gaps, research must address:
- Access to Genetic Testing: Ensuring underserved populations can benefit from screening and interventions.
- Culturally Sensitive Communication: Explaining genetic risks in clear, respectful language.
- Policy and Reimbursement: Aligning healthcare systems to cover precision medicine services.
By focusing on equity, Britain’s sequencing release can serve as a model for global genetic initiatives.
Conclusion
Britain’s largest-ever genetic sequencing release from the UK Biobank marks a turning point in genomic research. With whole-genome data for millions, scientists will unlock new disease genes, advance personalized medicine, and guide public health strategies. Yet such promise comes with responsibilities: protecting privacy, ensuring diversity, and building infrastructure that turns data into discovery. International partnerships and ethical safeguards will be key. As researchers prepare for this data tsunami, the world stands ready to harness these insights—ushering in a new era where the blueprint of life guides better health for all.