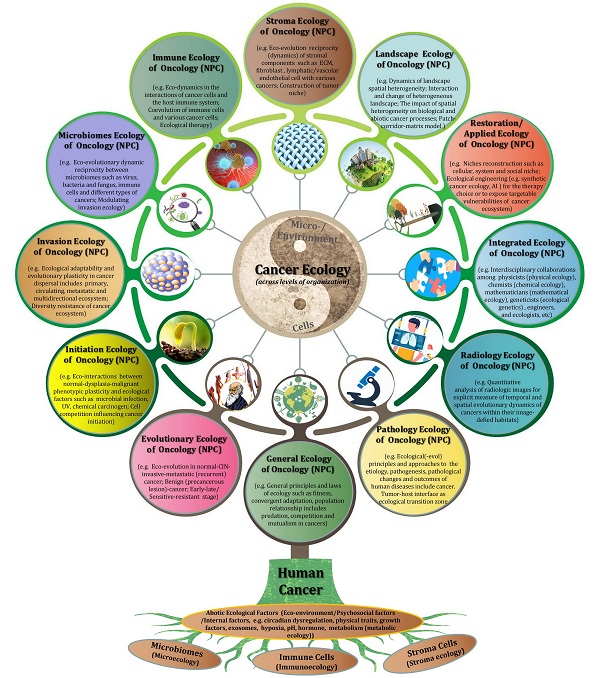
Introduction
Cancer is a complex disease characterized by uncontrolled cell growth and proliferation. One of the remarkable hallmarks of cancer cells is their ability to reprogram their metabolic pathways to meet the demands of rapid proliferation. Understanding these metabolic adaptations has been a focal point in cancer research, offering insights into potential targeted therapies.
Altered Glucose Metabolism
Cancer cells exhibit a preference for utilizing glucose, a phenomenon known as the Warburg effect. Unlike normal cells that primarily rely on oxidative phosphorylation for energy production, cancer cells favor glycolysis even in the presence of oxygen. This shift allows them to generate ATP and build macromolecules required for growth at an accelerated rate. The upregulation of key glycolytic enzymes, such as hexokinase and pyruvate kinase, fuels this process.
Amino Acid Metabolism
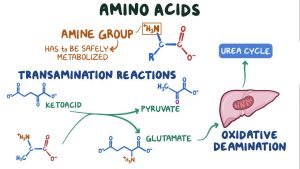
Another crucial adaptation in cancer cells involves alterations in amino acid metabolism. Cancer cells exhibit increased uptake of amino acids, particularly glutamine. Glutamine serves as a versatile nutrient, providing a carbon source for the tricarboxylic acid (TCA) cycle and contributing to the synthesis of nucleotides and lipids essential for cell proliferation.
Lipid Metabolism
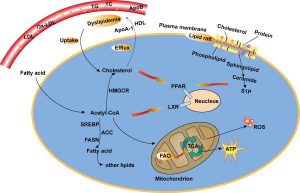
The rewiring of lipid metabolism is also a significant feature of cancer cells. Enhanced lipid biosynthesis and increased uptake of lipids from the microenvironment support the formation of cellular membranes and signaling molecules crucial for sustaining rapid proliferation. Enzymes involved in lipid synthesis pathways, such as fatty acid synthase (FASN), are often upregulated in cancer cells.
Targeting Metabolic Vulnerabilities
Exploiting the metabolic dependencies of cancer cells has emerged as a promising avenue for targeted therapies. Several strategies aim to disrupt these altered metabolic pathways, selectively targeting cancer cells while sparing normal cells.
Inhibiting Glycolysis
Therapies targeting glycolysis aim to inhibit key enzymes involved in this pathway. Small molecules and inhibitors targeting enzymes like hexokinase or pyruvate kinase have shown promise in preclinical studies. Additionally, drugs targeting glucose transporters on cancer cell membranes hinder glucose uptake, impeding their energy production.
Glutamine Targeting
Disrupting glutamine metabolism has gained attention as a potential therapeutic approach. Inhibitors targeting glutaminase, an enzyme crucial for converting glutamine to glutamate, have demonstrated efficacy in preclinical models, limiting cancer cell proliferation by depriving them of this essential nutrient.
Lipid Metabolism Inhibition
Strategies aimed at inhibiting lipid metabolism pathways, such as targeting FASN or acetyl-CoA carboxylase (ACC), have shown promise in preclinical studies. Inhibiting these enzymes disrupts the synthesis of lipids vital for cancer cell growth, leading to reduced proliferation.
Conclusion
The intricate reprogramming of metabolic pathways in cancer cells provides a unique opportunity for targeted therapeutic interventions. By exploiting these metabolic vulnerabilities, researchers are paving the way for innovative treatment strategies that specifically target cancer cells while minimizing damage to healthy tissues. However, challenges persist in translating these promising preclinical findings into effective clinical therapies. Further research is needed to better understand the complexities of metabolic adaptations in various cancer types and their interplay with the tumor microenvironment. Deciphering the metabolic alterations in cancer cells has not only deepened our understanding of tumor biology but also holds immense potential for the development of more effective and targeted cancer therapies. The ongoing exploration of these metabolic pathways continues to fuel optimism in the quest for more precise and efficient treatments, offering hope for improved outcomes in cancer patients.