Thermodynamics is the branch of physics that deals with the relationships between heat, energy, and work. Understanding thermodynamics is crucial as it forms the basis of various scientific and engineering principles. In this article, we’ll delve into the fundamental concepts of thermodynamics to provide you with a comprehensive understanding of this fascinating field.
Introduction to Thermodynamics
What is thermodynamics?
Thermodynamics is the study of energy and its transformations. It explores how energy is transferred between different forms and how it affects the behavior of matter. From engines to refrigerators, thermodynamics governs the functioning of various systems.
Importance of understanding thermodynamics
A solid grasp of thermodynamics is essential in fields such as engineering, chemistry, and environmental science. It helps in designing efficient systems, predicting natural phenomena, and developing sustainable technologies.
Basic Concepts
Energy and its forms
Energy exists in various forms such as kinetic, potential, thermal, and chemical energy. Understanding these forms and their interconversion is fundamental to thermodynamics.
Laws of thermodynamics
The laws of thermodynamics lay down the basic principles governing energy and its transformations. They provide the foundation for understanding the behavior of thermodynamic systems.
Temperature and Heat
Understanding temperature
Temperature is a measure of the average kinetic energy of particles in a substance. It plays a crucial role in determining the direction of heat flow and the equilibrium state of a system.
Heat transfer mechanisms
Heat can be transferred through conduction, convection, and radiation. Each mechanism has its unique characteristics and applications in various scenarios.
Work and Energy
Definition of work
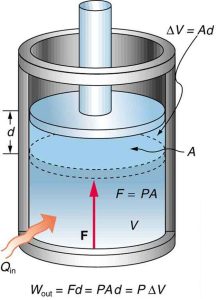
In thermodynamics, work is defined as the transfer of energy caused by a force acting through a distance. It is a crucial aspect of many thermodynamic processes, including mechanical work and boundary work.
Energy conservation principles
The conservation of energy principle states that energy cannot be created or destroyed, only transformed from one form to another. This principle underpins all thermodynamic processes.
Thermodynamic Systems
Open, closed, and isolated systems
Thermodynamic systems can be classified based on the exchange of matter and energy with their surroundings. Understanding these system types is essential for analyzing thermodynamic processes.
Boundary work and its significance
Boundary work refers to the work done during a change in the volume of a system. It is an important aspect of thermodynamic processes involving gases and fluids.
Properties of Substances
State variables and properties
Properties such as pressure, temperature, and volume define the state of a substance. These state variables play a crucial role in characterizing thermodynamic systems.
Equilibrium and stability
Thermodynamic equilibrium is a state of balance where no spontaneous changes occur. Understanding equilibrium conditions is essential for analyzing thermodynamic processes.
Thermodynamic Processes
Types of processes
Thermodynamic processes can be classified based on the changes in the state variables of a system. Common types include isothermal, adiabatic, and isobaric processes.
Graphical representations
PV diagrams are graphical representations of thermodynamic processes, showing the relationship between pressure and volume. They provide valuable insights into the behavior of thermodynamic systems.
Entropy and Entropy Changes
Introduction to entropy
Entropy is a measure of the disorder or randomness of a system. It plays a crucial role in determining the direction of spontaneous processes in thermodynamics.
Entropy change in reversible and irreversible processes
Entropy change is related to the heat transfer and temperature change in a system. Understanding entropy changes helps in analyzing the efficiency of thermodynamic processes.
Heat Engines and Refrigerators
Efficiency and coefficient of performance
Efficiency measures the effectiveness of heat engines in converting heat into work, while the coefficient of performance quantifies the effectiveness of refrigeration systems.
Carnot cycle and its significance
The Carnot cycle is a theoretical cycle that represents the most efficient heat engine possible. It serves as a benchmark for evaluating the performance of real heat engines.
Applications of Thermodynamics
Engineering applications
Thermodynamics finds applications in various engineering fields, including mechanical, chemical, and aerospace engineering. It is essential for designing efficient engines, turbines, and refrigeration systems.
Environmental implications
Understanding thermodynamics is crucial for addressing environmental challenges such as climate change and energy sustainability. It helps in developing renewable energy technologies and optimizing energy usage.
Challenges and Future Developments
Current challenges in thermodynamics
Despite significant advancements, there are still challenges in areas such as achieving higher efficiency in heat engines and developing sustainable energy solutions.
Emerging trends and research areas
Research in thermodynamics is focused on areas such as nanotechnology, renewable energy, and quantum thermodynamics. These emerging trends hold promise for addressing current challenges and unlocking new possibilities.
Conclusion
In conclusion, thermodynamics is a fascinating field that forms the foundation of various scientific and engineering principles. By understanding the fundamental concepts of thermodynamics, we gain insights into the behavior of energy and matter, enabling us to design efficient systems and address complex challenges.
FAQs
- What are the four laws of thermodynamics?The four laws of thermodynamics describe the fundamental principles governing energy and its transformations. They include the zeroth law, the first law (conservation of energy), the second law (entropy), and the third law (entropy of perfect crystals).
- How is thermodynamics applied in everyday life?Thermodynamics has numerous applications in everyday life, from heating and cooling systems to cooking and transportation. Understanding thermodynamics helps in designing efficient appliances, optimizing energy usage, and solving practical problems.
- What is the significance of entropy in thermodynamics?Entropy is a measure of the disorder or randomness of a system. It plays a crucial role in determining the direction of spontaneous processes and the efficiency of thermodynamic systems.




