Understanding the intricate relationship between immunometabolism in parasitic infections and cancer unveils intriguing parallels and disparities in immune responses and metabolic alterations. Exploring these similarities and differences not only illuminates the complexity of host-pathogen interactions but also unveils potential therapeutic avenues that harness these shared mechanisms.
Immune Response
Parasitic Infections
In parasitic infections, the immune response typically involves a Th2-skewed response characterized by the activation of eosinophils, mast cells, and production of cytokines like IL-4, IL-5, and IL-13. This response aims to expel parasites through mechanisms like antibody-mediated cytotoxicity and eosinophilic degranulation. However, chronic parasitic infections can induce immune exhaustion and regulatory T cell responses, dampening the host’s ability to clear the infection.
Cancer
Contrastingly, cancer often manipulates the immune system, creating an immunosuppressive microenvironment. Tumors exploit immune checkpoints like PD-1/PD-L1 to evade immune surveillance and activate regulatory T cells and myeloid-derived suppressor cells, inhibiting cytotoxic T cell responses. Additionally, tumors promote an immunosuppressive milieu by releasing factors like TGF-β and IL-10.
Metabolic Changes
Parasitic Infections
Parasites often modulate host metabolism to meet their energy demands. For instance, helminths can induce host metabolic alterations by diverting nutrients and energy sources, leading to changes in lipid metabolism and glucose utilization. Host cells may shift to anaerobic glycolysis, akin to the Warburg effect observed in cancer cells.
Cancer
Cancer cells exhibit metabolic reprogramming, favoring aerobic glycolysis even in the presence of oxygen (the Warburg effect). This metabolic shift supports rapid proliferation by providing substrates for macromolecule biosynthesis. Additionally, cancer cells alter pathways like the pentose phosphate pathway and glutamine metabolism to sustain growth.
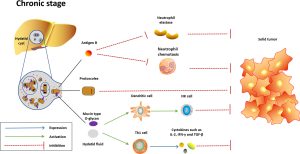
Therapeutic Avenues
Targeting Metabolic Pathways
Given the metabolic similarities, therapeutic strategies targeting shared metabolic vulnerabilities could be promising. Drugs inhibiting glycolytic enzymes or glutamine metabolism might impede both cancer and parasite growth. For instance, inhibitors of lactate dehydrogenase or glutaminase have shown efficacy in limiting cancer progression and might have potential against parasites.
Immunomodulation
Immunotherapies designed to overcome immune evasion mechanisms hold potential against both cancer and parasitic infections. Immune checkpoint inhibitors, which have revolutionized cancer treatment, could be repurposed to enhance immune responses in chronic parasitic infections by restoring T cell function.
Combination Therapies
Combining immunotherapy with metabolic interventions might yield synergistic effects. For instance, coupling immune checkpoint blockade with inhibitors targeting specific metabolic pathways could enhance antiparasitic or anticancer immune responses while impeding the energy supply crucial for parasite or tumor growth.
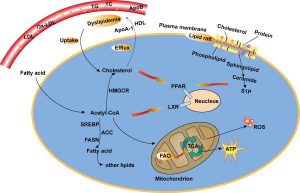
Precision Medicine
Advances in precision medicine offer tailored approaches. Identifying metabolic and immunological signatures in patients with parasitic infections or specific cancers could aid in devising personalized therapeutic regimens, maximizing treatment efficacy while minimizing adverse effects.
Conclusion
Immunometabolism in parasitic infections and cancer exhibits intriguing parallels in immune responses and metabolic alterations. Leveraging these similarities presents promising therapeutic opportunities. By targeting shared metabolic vulnerabilities and employing immunomodulatory approaches, innovative therapies may emerge to combat both parasitic infections and cancer, potentially revolutionizing treatment strategies in these disparate yet interconnected fields. Understanding these intricacies not only aids in therapeutic development but also sheds light on the fundamental mechanisms governing host-pathogen interactions and tumorigenesis.