Introduction
Metabolic reprogramming in cancer therapy has emerged as an intriguing avenue for combating this complex disease. Tumors exhibit altered metabolic processes, a hallmark known as the Warburg effect, where they favor glycolysis even in the presence of oxygen. This phenomenon highlights the potential of targeting specific metabolic pathways in tumor cells as a therapeutic strategy.
Understanding Metabolic Reprogramming in Cancer
Cancer cells display a remarkable ability to rewire their metabolic pathways to sustain rapid proliferation, invasion, and survival. Unlike normal cells that primarily rely on oxidative phosphorylation for energy production, cancer cells exhibit a preference for glycolysis, producing ATP even in oxygen-rich environments. This altered metabolism fuels the anabolic demands of cancer cells, providing not only energy but also intermediates for biosynthetic processes crucial for their uncontrolled growth. Targeting these metabolic alterations presents a promising strategy for cancer therapy. For instance, inhibiting glycolysis or redirecting cancer cells towards oxidative phosphorylation has been investigated to impair tumor growth and survival. Several key metabolic pathways have shown potential in this endeavor.
Targeting Glycolysis
Glycolysis, the initial step in glucose metabolism, plays a pivotal role in cancer progression. Inhibiting enzymes involved in glycolysis, such as hexokinase and phosphofructokinase, has demonstrated efficacy in preclinical studies. Small molecules targeting these enzymes show promise in suppressing tumor growth by disrupting the energy supply essential for cancer cell proliferation. Additionally, lactate dehydrogenase (LDH), responsible for converting pyruvate to lactate, is upregulated in many cancers. Targeting LDH isoforms may not only hinder lactate production but also disrupt the tumor microenvironment, rendering it less favorable for cancer progression.
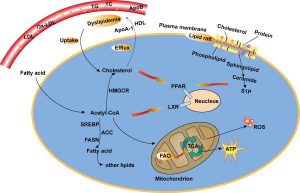
Modulating Mitochondrial Function
Mitochondria, the powerhouse of cells, play a crucial role in energy production. Cancer cells often display dysfunctional mitochondria, leading to an altered metabolic state. Approaches aimed at restoring mitochondrial function or inducing mitochondrial apoptosis have gained attention as potential therapeutic strategies. Metformin, a drug commonly used in diabetes management, has garnered interest due to its ability to modulate mitochondrial metabolism. Studies suggest its potential in inhibiting complex I of the mitochondrial electron transport chain, leading to impaired cancer cell proliferation.
Targeting Amino Acid Metabolism
Beyond glucose, cancer cells utilize alternative nutrients like amino acids to sustain their rapid growth. Targeting amino acid metabolism, particularly glutamine and serine metabolism, has shown promise. Glutaminase inhibitors, for instance, have exhibited anticancer effects by disrupting the synthesis of macromolecules crucial for tumor growth.
Emerging Therapeutic Approaches
In recent years, emerging strategies such as immunometabolism have gained attention. Modulating the metabolic pathways within immune cells to enhance antitumor immune responses represents a novel therapeutic approach. Immunotherapies combined with metabolic inhibitors aim to reprogram the immune cells’ metabolism to enhance their cytotoxic activity against cancer cells.
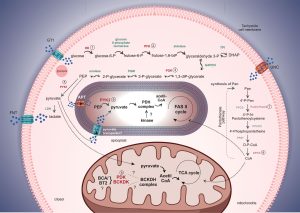
Challenges and Future Directions
Despite the promising potential of metabolic reprogramming in cancer therapy, several challenges remain. Tumor heterogeneity, adaptive resistance to metabolic inhibitors, and potential side effects on normal cells are significant hurdles. Additionally, translating preclinical success into clinically effective therapies requires further investigation and optimization. Future directions involve the development of targeted therapies that selectively disrupt cancer-specific metabolic vulnerabilities while minimizing adverse effects on healthy tissues. Combination therapies, integrating metabolic inhibitors with standard treatments like chemotherapy or immunotherapy, hold promise in overcoming resistance and improving clinical outcomes.
Conclusion
Targeting specific metabolic pathways in cancer cells presents a compelling therapeutic approach. Understanding the intricacies of metabolic reprogramming and its influence on tumor growth has led to the development of innovative strategies with the potential to revolutionize cancer treatment. Continued research and clinical advancements in this field hold immense promise in shaping the future of cancer therapy.